
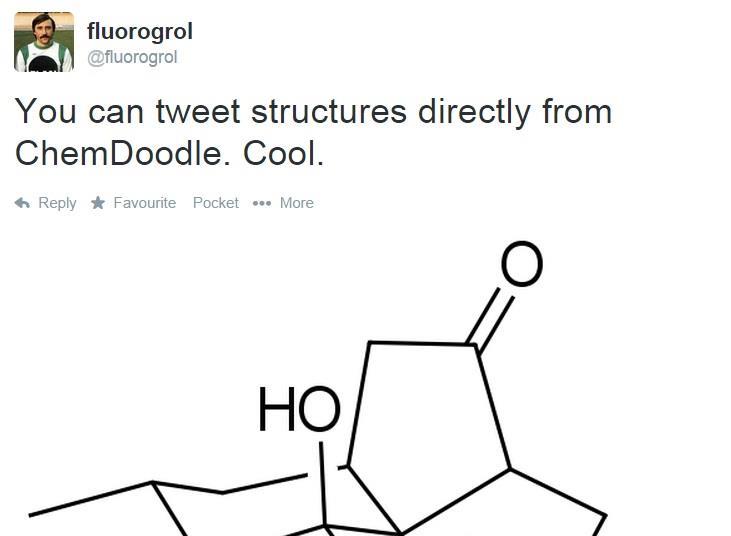
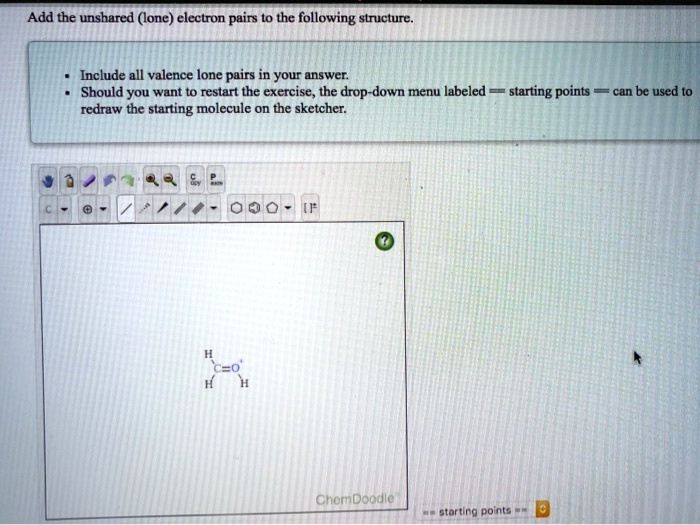
The ChemDoodle Web Components library is a pure JavaScript chemical graphics and cheminformatics library derived. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or backbone, of the macromolecules. a class of organic chemical compounds composed only of the elements carbon and hydrogen. carbon has the highest valence 4 from those 3 elements in methanol. Find the covalent bonds that are formed between carbon atoms. The second word tells you the second element and how many atoms there are in. publishers, and other vendors across the chemical information community. In some cases, the central atom can form more than other atoms connected to it. For unlimited access, please consider supporting the ChemDoodle team with a. Now that we understand hybridization states let's do a couple of examples all right and so we're going to identify the hybridization States and predict the geometries for all the atoms in this molecule except for hydrogen and so let's start with this carbon right here so the fast way of identifying a hybridization state is to say ok that carbon has a double bond to it therefore it must be sp2 hybridized and if it's sp2 hybridized we know the geometry around that carbon must be trigonal planar with bond angles approximately 120 degrees this carbon over here right is also I also has a double bond to it so it's also sp2 hybridized with trigonal planar geometry all right let's move to this carbon right here so that carbon has only single bonds around it and the fast way of doing it is it is if you see all single bonds it must be sp3 hybridized and if that carbon is sp3 hybridized we know the geometry is tetrahedral all right so tetrahedral geometry with an ideal bond angles of 109. See how many Carbon atoms are in the chain. The Chemistry Add-in supports ChemDoodle web, a fully featured structure editor.


 0 kommentar(er)
0 kommentar(er)
